x

Showroom
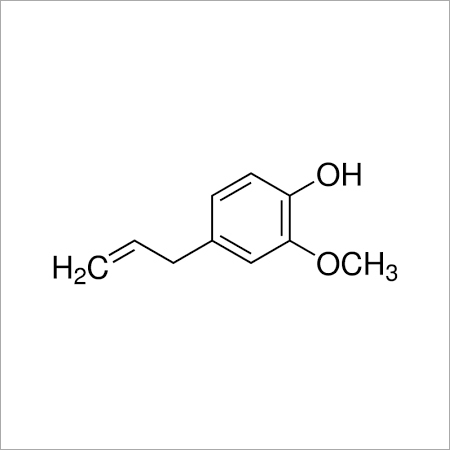
Aromatic Compounds have a sooty yellow flame because of their high carbon-to-hydrogen ratio. They are helpful as solvents for other nonpolar chemicals because they are often unreactive. Aromaticity refers to the peculiar stability of these molecules. These compounds are very effective and economical to use.
Essential Oils are those oils which are used to provide a proper relaxment to our body and mind. These oils are very much liked and highly appreciated by people, in the market. These oils are easy to use and protects you from getting ill or any other kind of diseases.
 |
MISRI FUMET PVT. LTD.
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese